 "1.21 JIGGA WATTS!!!" (121jiggawatts)
"1.21 JIGGA WATTS!!!" (121jiggawatts)
04/07/2015 at 09:40 • Filed to: None
 0
0
 6
6
 "1.21 JIGGA WATTS!!!" (121jiggawatts)
"1.21 JIGGA WATTS!!!" (121jiggawatts)
04/07/2015 at 09:40 • Filed to: None |  0 0
|  6 6 |
That can bend and be drilled through while still working.
Click !!!error: Indecipherable SUB-paragraph formatting!!! to read the full story, but in a nutshell, the battery can be charged in about a minute, and uses aluminum anodes with graphite cathodes to create a working prototype. Obviously, capacity isn't mentioned this early in the game, but I imagine that that will be fairly easy to scale up.
!!! UNKNOWN CONTENT TYPE !!!
The one major hurdle is voltage output. While it puts out about 2 volts after thousands of recharges (which is higher than most other aluminum-ion batteries), that's only about half of what a lithium-ion smartphone battery puts out. They do show in the video that pairing two batteries can power a smartphone, proving that that jump to smartphone level batteries could be closer than we all think.
Also, think of the large-scale applications. This innovation could be the tech necessary for Tesla Motors to make the jump into the rural/long-distance car market by making charge times shrink even smaller than what they are now. Paging Elon Musk ...
 Quadradeuce
> 1.21 JIGGA WATTS!!!
Quadradeuce
> 1.21 JIGGA WATTS!!!
04/07/2015 at 09:55 |
|
*gigawatts.
This isn't a Will Smith music video.
 1.21 JIGGA WATTS!!!
> Quadradeuce
1.21 JIGGA WATTS!!!
> Quadradeuce
04/07/2015 at 09:59 |
|
I know it's a gigawatt, but I hear jiggawatt. I listen to Doc...
 Racescort666
> 1.21 JIGGA WATTS!!!
Racescort666
> 1.21 JIGGA WATTS!!!
04/07/2015 at 10:08 |
|
So the advantages are: charge cycle (time), cost, and safety? Speaking as an automotive engineer, energy density has always been the biggest issue with batteries.
Cost can somewhat be driven down by economies of scale (the more you produce of a particular part, the cheaper the individual parts are). This is the idea behind the Gigafactory, or my assumption anyway. Although, if the cost of these batteries were so much cheaper than their lithium counterparts, despite the lack of performance, it would be incentive for the industry to use them.
Weight is (would be) still an issue but I think if batteries were something like 1/10th the cost, even at half the performance, it could be worth it.
 1.21 JIGGA WATTS!!!
> Racescort666
1.21 JIGGA WATTS!!!
> Racescort666
04/07/2015 at 10:21 |
|
I would agree with those advantages. I didn't see anywhere in the article (and my browser wouldn't let me access the research paper) anything about energy density. I would also argue that lithium availability versus aluminum would drive cost and/or research.
Lithium-sulfur batteries are said to have a 500 Wh/kg energy density while aluminum-ion batteries are stated to have 1000 Wh/kg energy densities, both which are above lithium-ion densities. (Caution: linked articles are Wikipedia. "Dammit Jim, I'm a Mechanical Engineer, not an Electrical Engineer")
I'm finding anywhere from 200-400 Wh/kg energy densities for lithium-ion, across multiple Wikipedia pages, so take everything here with a grain of salt, as us engineers should.
 Racescort666
> 1.21 JIGGA WATTS!!!
Racescort666
> 1.21 JIGGA WATTS!!!
04/07/2015 at 10:33 |
|
I think you're referring to the citation on the Wikipedia page? It's literally a PDF of a powerpoint presentation. It's got this neat table though
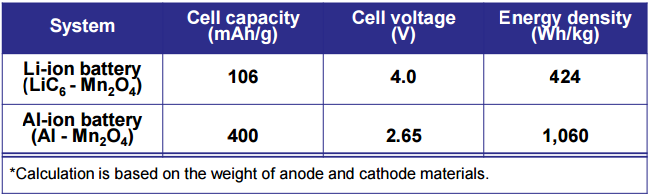
 1.21 JIGGA WATTS!!!
> Racescort666
1.21 JIGGA WATTS!!!
> Racescort666
04/07/2015 at 10:41 |
|
I was, but was lazy, so I just linked Wikipedia. I misread the aluminum one a little too. The page below the slide you screenshot says the theoretical energy density for Al-ion is 8140 Whr/kg vs. Li-ion's 1462 Whr/kg. So now, I'm a little confused...